Pharmacometrics uses mathematical models of biology, pharmacology, disease, and physiology to describe and quantify beneficial or adversary interactions between drugs and humans.
What is Pharmacometrics?
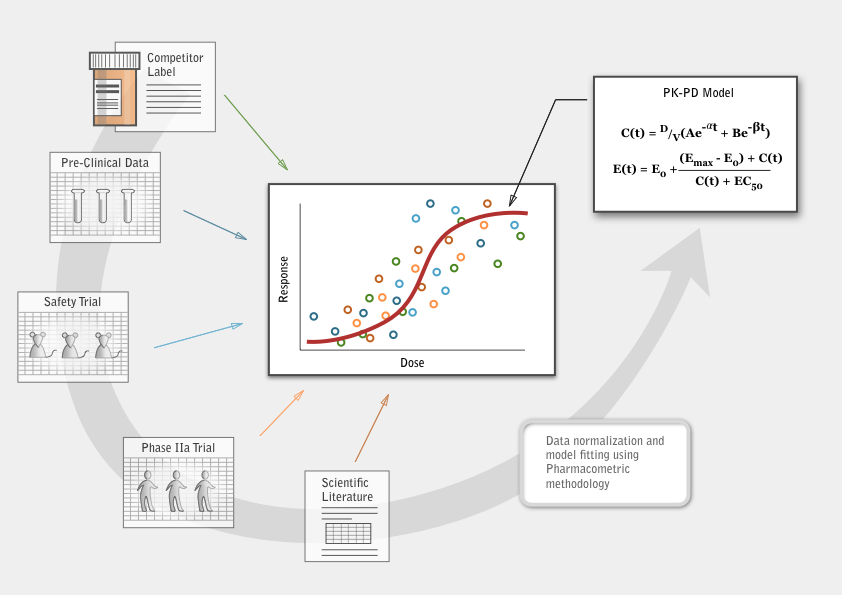
Pharmacometrics uses models to describe the beneficial and adverse effects of a drug therapy on a patient. Data extracted from pre-clinical studies, clinical studies, scientific literature and competitor regulatory filings are integrated, building a complete data package describing the characteristics of a given lead compound. From this integrated data set, mathematical models of exposure and response are built and qualified. These models become a representation of what is known about the compound’s behavior.
Why Pharmacometrics?
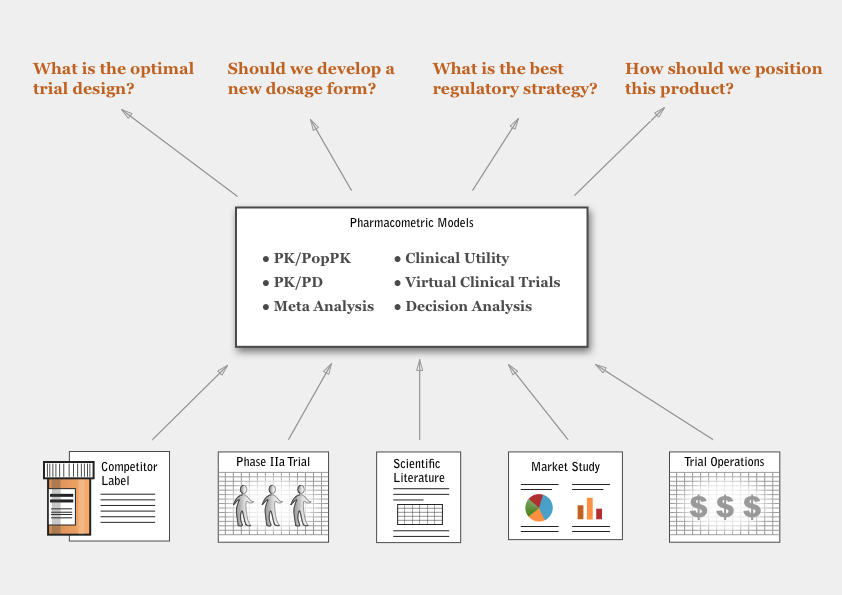
By combining different types of models with a simulation step, a pharmacometrician brings quantitative insight to the strategic decisions arising in commercial drug development.
“The single-most important strength of pharmacometric analysis is its ability to integrate knowledge across the development program and compounds, and biology.”
– U.S. FDA
your business value? Get in touch for an online meet-up.