Clinical Pharmacology investigates the interaction between drugs and humans.
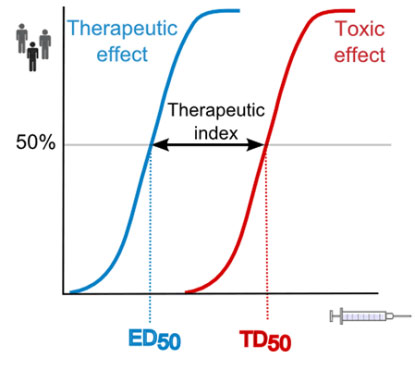
This requires in-depth knowledge on many aspects of drug development, i.e. (bio)chemistry, formulation development, (pre)clinical absorption/distribution/metabolism/excretion (ADME), pharmacokinetics (PK), pharmacodynamics (PD), pharmacogenomics and drug-drug interactions (DDI).
The ultimate goal is to deliver the right dose to the right patient. By applying quantitative approaches (pharmacometrics, non-compartmental analysis (NCA)) and regulatory requirements, Clinical Pharmacology can determine the most efficient strategy within drug development.
Clinical Pharmacology applications can be…
- Dose selection from First In Human (FIH) to Phase 3
- Development & strategize the Clinical Pharmacology plan including:
- FIH
- bioequivalence (BE)
- food effect
- drug-drug interactions (DDI)
- thorough QT (TQT)
- special populations PK
- ADME studies
- Provide PK support for biopharmaceutical decisions throughout drug development
- Contribute to regulatory submissions (i.e. briefing books, summary documents such as 2.7.1 and 2.7.2) and meetings
- Support pediatric drug development (PSP or PIP)
- Innovative clinical trial design and clinical trial simulations
- PK analysis, interpretation and reporting of PK results from clinical trials
your business value? Get in touch for an online meet-up.




