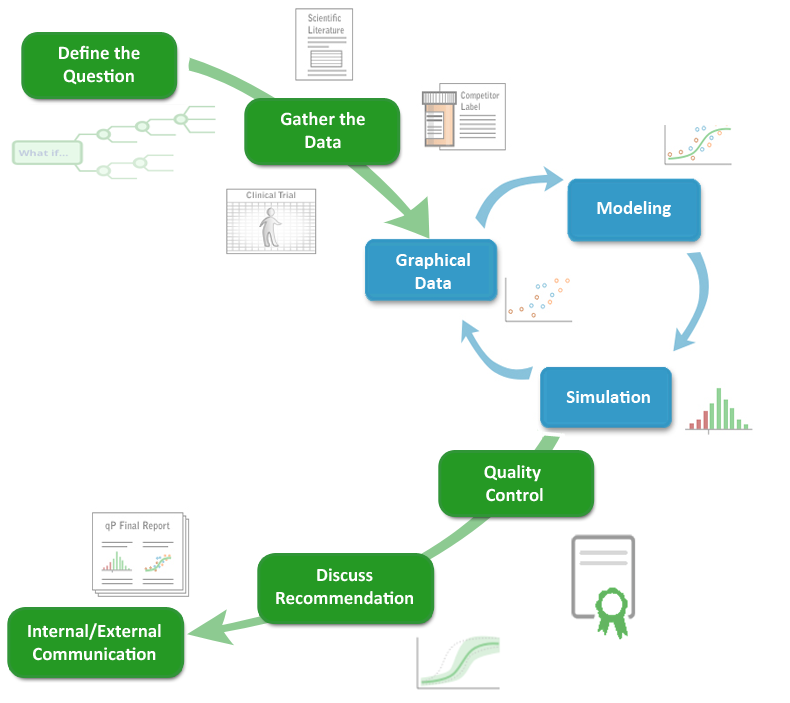
The qPharmetra process is disciplined and transparant, providing clients with a clear view of the modeling approach, complete documentation and compelling reports and graphics.
Though each project is unique, our standardized approach allows qPharmetra’s clients to benefit from institutional knowledge within the firm and a quality assurance process that is followed at each step. The result is efficient, consistent, quality output on every project.
Much of qP Analysis has been automated to save time and to allow consultants to focus on areas where our creativity will provide the most benefit: the thornier issues, the application of new techniques, and the creative process of developing better medicines, not just quantifying data you already have.