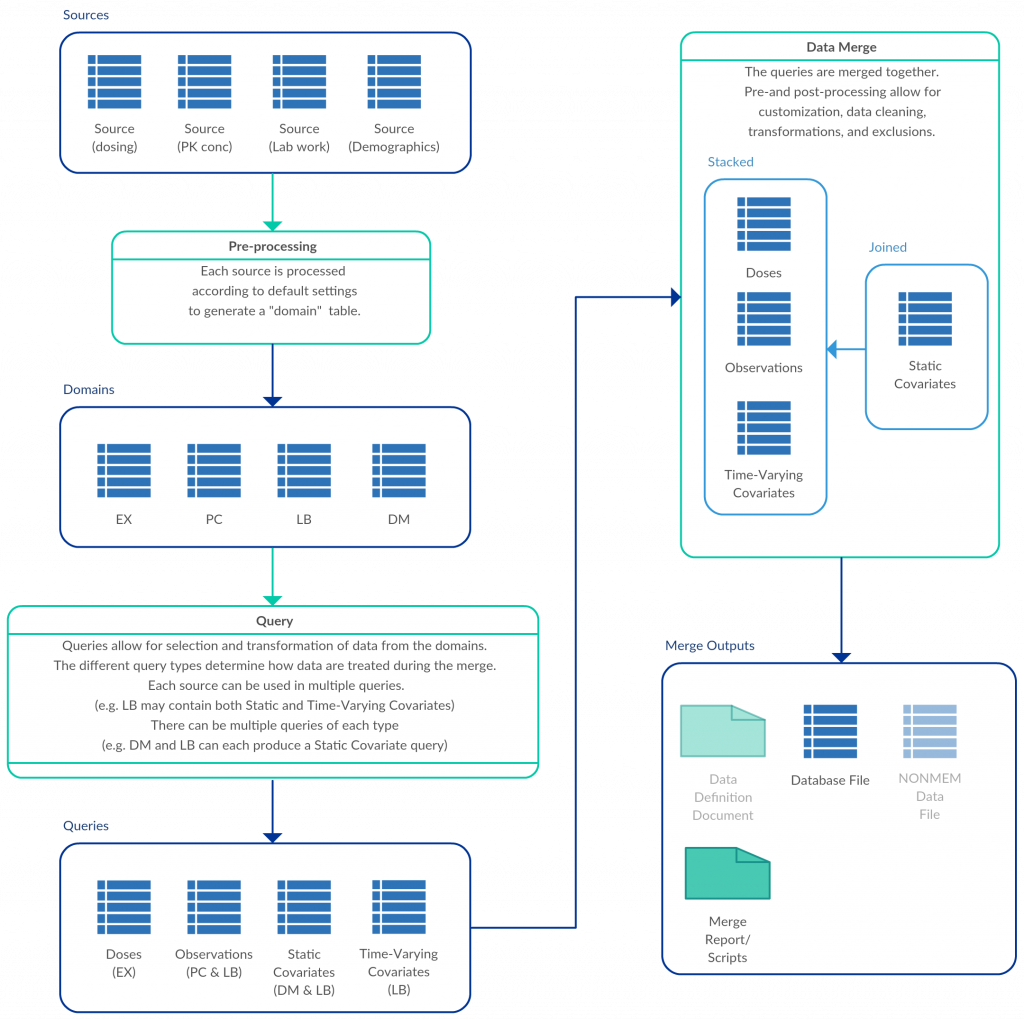
An R package for building pharmacometric data sets. PMDatR distills decades of expert knowledge regarding the intricate needs of pharmacometric analysis datasets.
PMDatR builds on top of popular R packages such as dplyr[1] and tidyr[2], so much of the syntax is well known and documented. The key features that facilitate data work flows include:
- functions for transforming, reformatting (such as automatically unstacking covariate columns in result domains like LB), and verifying inputs
- common transformations such as filling forward
- computation of ADDL dosing
- unit aware columns and transformations;
- automated code generation from a settings file.
Some common transformations include:
- automatic conversion of date/time formats
- change from baseline
- time after dose and similar calculations
- filling of missing covariate values.
PMDatR can be used directly from R scripts as well as automated through a custom Graphical User Interface (GUI), enabling a flexible, customizable, repeatable, templatized workflow.